How gluten gives its structure to bread
First of all, let me remind you what gluten is ??
In wheat flour, there are two very important proteins: gliadins and glutenins. These two proteins can combine with each other and with water and thus constitute a network.
Today, only 4 cereals contain enough gluten to be able to give flour that can be used to make bread: rye, oats, wheat anf barley.
If you want to use another type of flour, you'll have to make a mix with flour containing gluten, or find other additives that can replace it.
Ok, now we know what gluten is. But how does it give its structure to bread ?
1) When water is added to the flour, water combines with proteins (gliadin and glutenin) to form a network.
2) This network isn't well organized at first, but when the dough is mixed, longer strands of gluten appear.
During kneading, the network becomes stronger and more elastic.
3) While baking, gas is released from the yeast. For the inside of the loaf to be light, it is important that this gas should not be released during the baking.
That's the role of gluten: it creates a strong airtight network, which keeps the gas inside.
At the end of baking, the network is solid and you have bread.
" Bon appétit ! "
What happens when you beat egg whites?
What happens when you beat egg whites?
Have you ever wondered what happens when you beat egg whites? Have you ever succeeded in making egg whites? Your chocolate mousse is more a liquid than a mousse? So this article is for you.
This is the how to make egg whites!
When you beat egg whites, the aim of the operation is to incorporate air bubbles into the egg white. In fact the egg whites is constituted of a water-protein solution. When the eggs whites are beaten, it creates two kinds of stress in the solution. The first physical effect creates a force which unfolds the protein molecules of the molecule. This process is called denaturation. The second effect is to put these proteins out of their natural state of equilibrium. So they enter in contact with air and water.
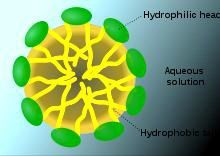
The proteins contained in the white egg are composed of amino acids. This kind of molecule has two physical opposite properties. One of the extremities is hydrophobic, that means that is water-fearing. The second extremity is hydrophilic so it’s water-loving. So before the first effect, all the proteins are curled up, the hydrophobic heads are packed in the center away from the water, and all the hydrophilic heads are outside close to the water. These kinds of molecules are called surface active agents.
So when an egg protein is up against an air bubble, one part of that protein is exposed to air and the second part is still in water. Indeed the proteins become uncurled, so the hydrophilic part is immersed in the water and the hydrophobic part is in the air. It creates bonds with each other forming a network that can hold the air bubbles in place. It’s called coagulation.
However if you introduce any form of fat, like cooking oils, your egg white will not beat up. Indeed the hydrophobic part will be attracted by the oils and so not by the air.
So if you don’t want your egg white to collapse, you have to stabilize this structure. You can use copper bowl to reinforce the bonds or add cream of tartar, which is an acid and will change the pH and stabilize your foam.
Now that you’re an expert! Let’s go cooking a chocolate mousse.
Triboluminescence in candies !
Triboluminescence is a phenomenon which appears when two pieces of a special material (sugar crystal for example) are stricken .
You can observe it with the LifeSavers candy because it's a sugar crystal.
Let's explain the phenomenon scientifically !
Stressing the crystal by biting into the candy makes the positive and negative charges separated, creating an electric field. The charges are accumulated and when it's enough the excited electrons recombine with the nitrogen molecules in the air then they emit light in the visible range, but also in the ultraviolet which you can't see. The color in your mouth is kind of blue .And don't worry it's not dangerous, you can eat as many candies as you want !
If you click on the video you can see what happens in your mouth, the crystal (or big rock) symbolises the candy. When the experimentor switches off the light you can see the light emitted by the crystal ! Do it yourself you just need a LifeSavers candy and a miror, enjoy the magics !
Triboluminescence in Quartz Crystal
 These are the candies which are the most efficient, unfortunaly they are not available in France :(
These are the candies which are the most efficient, unfortunaly they are not available in France :(
Quick presentation and aim of the blog
Hi there !
We are a group of 3 very smart engineers to be, who are going to help you impress your friends and familly with amazing knowledge about cooking science !
Hope you'll enjoy !